No molecular orbital diagram 10. the mo diagrams of no and no+ are similar (see lecture 15 for the Sf6 molecular orbital diagram mo diagram of no+
MO Diagram Of No
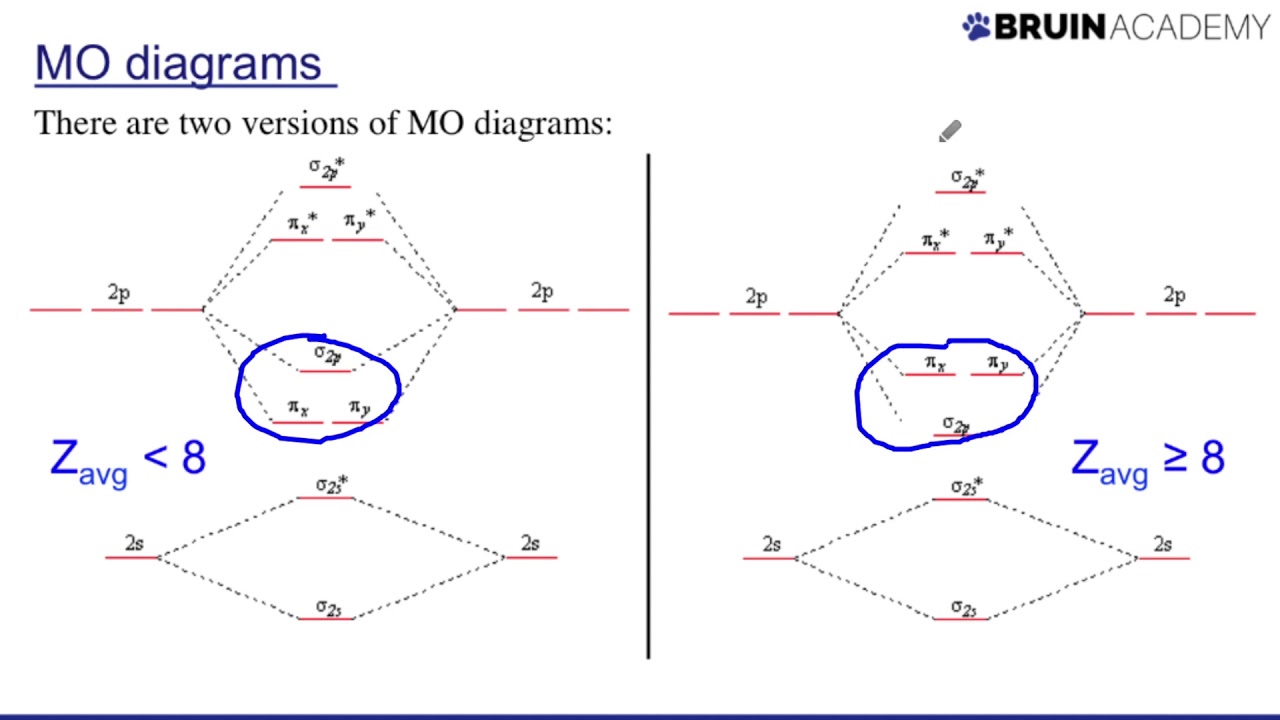
Mo diagram Mo diagram of no How to draw molecular orbital diagram of no
10. the mo diagrams of no and no+ are similar (see lecture 15 for the
Molecular orbital diagram for li2Solved use the mo diagram for no as a guide to answer the Is $ n{o^Orbital orbitals diagram molecular mo br2 draw configuration bond order chemistry o2 answer calculate explain electron socratic superimposed depictions original.
Paramagnetic diamagnetic chemistry orbital41 complete this molecular orbital diagram for cn Mo diagram for he2Nitrogen (diatomic).

Solved: chapter 12 problem 1p solution
3: molecular orbital diagram of no.Mo diagram diagrams molecular orbital theory No molecular orbital diagramMo diagrams.
Lecture 20 : no & no+ m.o. energy level diagramNo molecular orbital diagram In this schematic mo diagram for 1 n ( n = − , 0, + ), 1 − has the π14+ hbr mo diagram.

[diagram] d orbitals mo diagrams
Mo diagram noHow to draw overlapping of pure or hybridized orbitals for br2 and no+ 10. the mo diagrams of no and no+ are similar (see lecture 15 for the10. the mo diagrams of no and no+ are similar (see lecture 15 for the.
He2 mo diagram orbital molecular chemistry he theory diagramsMo diatomic molecules diagrams Mo molecular orbital diagram oxide n2o structure cl2 theory orbitals nitrogen diagrams nitric energy geometry molecule lewis hybridization example oxygenMo diagram for no+.
37 no+ molecular orbital diagram
[diagram] d orbitals mo diagrams10.3: beh₂ is linear and h₂o is bent Mo diagramsMo diagram.
Mo diagram for no+ .